Tree Diseases
Tree diseases are a significant threat to Mississippi’s trees. Diseases cause 45% of all mortality and degradation in our forests.
- Annosus Root Rot
- Brown Spot Needle Blight
- Fusiform Rust
- Hardwood Decay (Heart Rot)
- Laurel Wilt Disease
- Littleleaf Disease
- Loblolly Pine Decline
Annosus Root Rot

Hosts
Annosus root and butt rot is a commercially important disease of conifers. All southern pines are susceptible, but loblolly and slash pine are most severely affected.
Identification
Conks are often present in the litter at the base of dead or dying trees or tree stumps, or under root masses of windthrown trees. Conks, when fresh, are tan to brownish on the upper surface and white with tiny pores on the lower surface. They are rubbery and tough to tear. In the southern United States, conks are most common from December through March.
Damage
Damage from annosus root and butt rot may be scattered throughout a stand or in pockets of dead and dying pine trees called “infection centers.” Mortality is sometimes preceded by thinning and yellowing of the crown; however, some trees simply turn red and die. Trees in various stages of dying or death may suffer windthrow. Infected roots exhibit resin or pitch-soaking, and stringy root decay.
Biology
Annosus root and butt rot probably enters the stand when fungal spores land on fresh cut stump surfaces. The fungus grows through the remaining root system into nearby live trees via root grafts or contacts. Mortality usually begins 2 to 3 years after thinning and often ceases 5 to 7 years later. Damage increases with the sand content of the soil. Twelve inches (30 mm) or more of sand or sandy loam above a clay subsoil in a soil with good internal drainage is considered a high hazard site for tree mortality.
Control
Prevention and control strategies for annosus root rot include stump treatment, timing of thinnings, prescribed burns, and the manipulation of planting density.
Brown Spot Needle Blight

Photo by George Blakeslee, University of Florida.
Hosts
Brown spot needle blight, caused by the fungus Mycosphaerella dearnessii (syn. Scirrhia acicola), is common on longleaf pine seedlings within the natural range of longleaf pine, that is, within the Coastal Plain from North Carolina to Texas. The fungus also infects seedlings of slash, loblolly, and white pines in nurseries within or slightly beyond this area.
Distribution
Brown spot is found in nurseries in nine Southern States. It has also been reported on pines in plantations in nine additional States.
Damage
Infected seedlings are seldom killed, but severe defoliation reduces vigor, which, in turn, may result in poor survival and growth following outplanting.
Identification
Lesions may develop on secondary needles at any time, but they most commonly appear from May to October. Look for small, grayish-green spots, which become a straw-yellow color and then light brown with chestnut-brown margins. Spots coalesce, and the needle tissue dies beyond and between groups of spots. Needles with multiple lesions appear motted. The fungus can also infect the cut ends of pruned longleaf pine seedlings.
Biology
Ascospores, discharged during periods of rain, dew, and fog, are the principal means by which the brown spot fungus invades nursery beds. Ascospores are disseminated by the wind and initiate light, scattered infections, sometimes at great distances from the source. Conidia are produced in acervuli on the lesions resulting from ascospore infection. Conidia are disseminated short distances by rain splash and cause local buildup of the disease. Ascospores are produced on seedlings 2 to 3 months after the seedlings are infected. Both spore forms overwinter in dead and infected needle tissue.
Control/Prevention
Use superior seed that is resistant to the disease. Remove and destroy all infected seedlings and infected pines growing in and around the nursery. Remove clipped needles from nursery bed areas. Avoid pruning when it is raining or at any time when the seedlings are wet. Spray with Bordeaux mixture, maneb, or chlorothalonil, which are effective and registered for use on brown spot. Seedlings should be sprayed wit a foliar fungicide at 10- to 30-day intervals – depending upon the amount of rainfall – from the beginning of April through October. Begin spraying in the spring when the new secondary needles are 1 to 2 inches long. Four to six applications are usually sufficient. A seedling root-dip treatment in a 5-percent active ingredient benomyl-kaolin mixture prior to packing at the nursery or at the reforestation site is very effective in reducing brown spot in the field. This treatment is very economical and significantly improves both growth and survival of outplanted seedlings.
Fusiform Rust
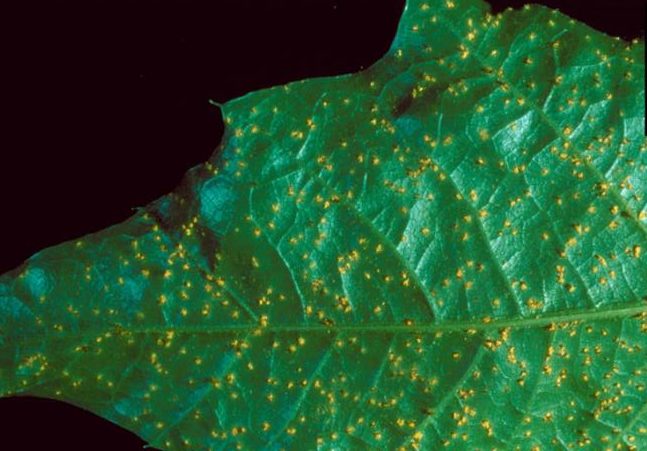
Hosts
In forest stands these diseases are of minor importance on oak (alternate host). However, they affect the aesthetic value of shade trees and ornamentals. Fusiform rust on pine (primary host) is the most important disease of pine in the Southeast.
Identification
Small yellow spots develop on the leaf surfaces in spring. Some defoliation may occur. Red, water and willow oaks are primarily affected. White oaks are seldom affected.
Biology
Leaf rusts require two hosts to complete their life cycle. Fungus spores (aeciospores) produced on pine galls are windblown and infect young oak leaves. Spores (urediospores) are produced on the oak leaf which reinfect oak. Spiny-like hairs (telial columns) on the lower oak leaf surface release teliospores which produce another spore stage (basidiospore) that infects pine. This infection results in a gall with aeciospores, and the cycle is complete.
Control
No control needed
Hardwood Decay (Heart Rot)

Heart rot is the single most important disease of merchantable hardwood timber in the South, causing 75% of these losses. Heart rot can affect all parts of the tree, but frequently occurs in the butt log where its impact on the value of the tree is greatest. In the earlier stages, damage is in degraded wood. Advanced decays cause hollows, tree breakage, and mortality.
Identification
Damage from heart rot is easily observed through physical evidence of hollows, rotten wood, irregular or lumpy stems, cankers, catfaces, scars, and fungus fruiting bodies on stems. Most begin at basal injuries, like those caused by fire and logging damage. Different fungi may behave differently in timber stands by rate of decay and spread, and host species attacked. Trees are predisposed to decay by wounding caused by insects, weather, fire, animals, and human activities such as logging.
Prevention
Minimize decay by growing species adapted to the site and by preventing wounding. Carefully supervise fire control and prevention, road building, logging and other forest activities. When clearcutting for coppice regeneration, stumps should be as close to the ground as possible.
Control
Once the decay process begins, there is no control. When thinning hardwood stands for regeneration, remove all diseased trees. If this is uneconomical, cut the tree and leave it on the ground to reduce inoculum dispersal. This also allows decay fungi to be overrun by decomposing fungi. Never put scar sealant over existing scars, because this may further promote decay.
Laurel Wilt Disease

Laurel wilt is a deadly disease of redbay (Persea borbonia) and other tree species in the Laurel family (Lauraceae).
Laurel wilt has been confirmed in the field on several other Lauraceous hosts including avocado (Persea americana), sassafras (Sassafras albidum), camphor (Cinnamomum camphora), and the endangered species pondberry (Lindera melissifolia) and pondspice (Litsea aestivalis).
Identification
The disease is caused by a fungus (Raffaelea lauricola) that is introduced into host trees by a non-native insect, the redbay ambrosia beetle (Xyleborus glabratus), that was first detected in the United States near Savannah, Georgia, in 2002. The beetle is believed to have been introduced in wooden crating material imported through the shipment of goods from its native range in southeast Asia.
The fungus plugs the water-conducting cells of an affected tree and causes it to wilt. Laurel wilt has caused widespread and severe levels of redbay mortality in the Southeastern coastal plain.
Symptoms
Trees with laurel wilt initially exhibit drooping foliage with a reddish purplish discoloration in a portion of the crown. The symptoms will gradually cover the entire crown and turn brown in color. Wilted leaves may remain on the infected trees for a year or more.
In the early stages of the disease, a wilting tree may not show any obvious signs of ambrosia beetle attack. Early attacked are inconspicuous and may happen on branches in the crown or on the stem. As the tree dies from the fungal infection, it is colonized by more ambrosia beetles which produce small dowels or “toothpicks” of sawdust protruding from the stem as they bore into the wood.
Distribution
Laurel wilt can spread in at least two ways: one is via the beetle’s natural reproduction and migration. A second way is through the sale and transport of beetle-infested wood, a result of redbay’s use as firewood and for outdoor grilling.
Littleleaf Disease

Hosts/Causing Factors
Littleleaf disease is the most important disease affecting shortleaf pine in the South. Loblolly pine is also affected, but to a lesser degree. Affected trees often die within 6 years of first symptom expression. This disease is caused by a complex of factors which include Phytophthora cinnamomi, heavy clay soil, and soil that is low in nitrogen. Also, Pythium spp. and nematodes often contribute to the damage. While the soil can be evaluated on site, laboratory analysis is required for confirmation of the fungi, nematodes, and nitrogen deficiency.
Identification
While the damage is to the roots, the obvious symptoms are seen in the crown. The first symptom is needle yellowing. New needles are shorter and fewer in number. Eventually, the crown looks sparse and often has a tufted appearance. A heavy crop of small, very persistent cones normally develops 2 to 3 years prior to tree death. Often there is a flush of epicormic branches on the bole of the tree.
Biology
This disease occurs on trees growing on low quality sites-such as old fields. For various reasons, including nitrogen depletion, poor aeration, and rootlet competition, the rate of new rootlet formation by the tree declines and the rate of loss resulting from the killing action of P. cinnamomi increases. On poor sites, infected trees showing early symptoms are expected to survive for about 6 years. On better sites they may persist 15 to 20 years.
Control
Losses can be minimized by salvage, favoring loblolly pine within its range, or, where silviculturally appropriate, converting to hardwood. In an urban or high value forest situation, a high nitrogen fertilizer can be used to delay mortality.
Loblolly Pine Decline

Pine decline is a disease complex resulting from the interactions of both biotic and abiotic stressors. Because of the emphasis in planting loblolly pines for timber production, this disease complex is most prevalent in loblolly pine plantations. Shortleaf pine, however, is also quite susceptible to this disease, while longleaf pine and other conifer species are less susceptible. Thus far, evidence shows that slash pine is not susceptible to the factors that result in pine decline. Affected pines expressing declining symptoms can succumb within two to three years. Pine decline usually exists with trees that are over 35 years of age, but can exist in trees as young as 12 years old.
Identification
A specific soil type is not generally associated with this disease; however, pines in predominately loam, sandy loam or sandy clay loam are quite susceptible. Recent prescribed burns, past agricultural practices and lower vegetation density are contributing factors to pine decline. The most significant factors to this disease complex, however, are abiotic conditions such as drought or storm damage. Symptomatic pines will have bark beetles and Leptographium fungi present in deteriorating roots. Infected primary roots will have blue-stain and resin-soaked lesions caused by the Leptographium fungi (L. truncatum, L. procerum, L. terebrantis, L. serpens, and L. huntii).
Control
Currently, the procedure to prevent pine decline is still being determined. Insecticides and fungicides are not available for controlling this disease complex. Since the infection and damage occur underground, methods for identifying are difficult. The best prevention method is to properly manage the pines based on the specific site. Know the management history of the stand. If the land was once managed for agriculture, then certain pine species are more likely susceptible to pine decline. Also, the soil type can be a factor. The particular soil type can determine the pine species that are ecologically adapted to the site. Slash pine is the least susceptible to this disease, but its natural range is very limited. Longleaf pine is also less susceptible to pine decline than loblolly pine, but its growing habitat is also quite site specific. The only guaranteed management recommendation is to convert the land area into a non-pine (hardwood stand or a pasture) stand.
For more information about tree diseases, contact comments@mfc.ms.gov.
